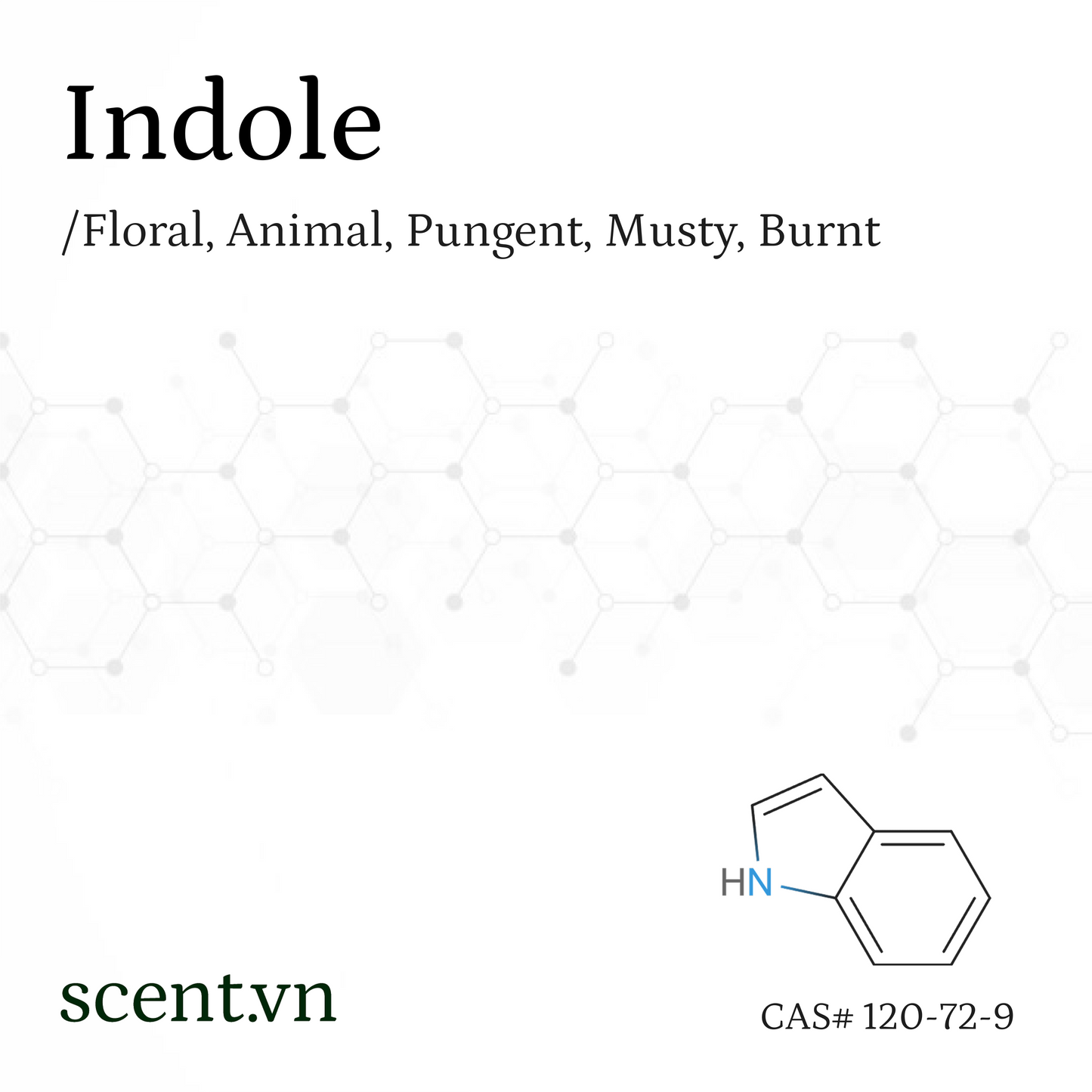
Indole Aroma Chemical
CAS# 120-72-9
Floral, Animal, Pungent, Musty, Burnt
Couldn't load pickup availability
Indole-also known in specialist circles by scientific names such as 2,3-benzopyrrole or ketole-is one of the most fascinatingly paradoxical fragrance materials in the world of scent. Its history is closely tied to the renowned chemist Adolf von Baeyer, who proposed its structure in 1866 while researching the indigo dye Indigo; the name indole itself is a meaningful blend of Indigo and Oleum. In nature, indole appears in a striking duality: it is both a key soul-note behind the intoxicating aroma of noble white flowers such as jasmine, bitter orange blossom, gardenia, and narcissus, and also a product of protein decomposition in animal waste, responsible for a characteristic foul odor.
To meet commercial demand, indole is typically isolated from coal tar or produced by chemical synthesis from phenylhydrazine and pyruvic acid. In appearance, indole exists as shiny, flaky crystals-white or colorless when pure-but it is highly sensitive and can readily shift toward pink or reddish-brown when exposed for long periods to light and air. Its most remarkable quality lies in its unpredictable olfactory profile: at high concentration it is sharp and penetrating, camphor-like and unpleasantly fecal, but when diluted to extremely low levels it transforms into a warm, seductive floral nuance. Thanks to this unique behavior, indole becomes an indispensable “golden key” for realistically recreating jasmine and tuberose in perfumery, and it is also used with great finesse in foods such as cheese, chocolate, and coffee to deepen and enrich flavor.
Description
Belonging to the animalic and white-floral family, indole typically functions from the heart note into the base, acting as an enhancer that lends a natural, lifelike beauty to the overall scent. Its olfactory character contains two dramatically opposing faces: in its neat form or at high concentration, it radiates a harsh, camphor-like sharpness reminiscent of mothballs or cockroach repellent, mixed with an unpleasant decomposed, fecal facet. However, the “magic” truly occurs only when diluted below 0.1%-at which point indole sheds its harshness and brings warmth, a fleshy sensuality, and vivid vitality to floral notes, making them feel real rather than artificially chemical.
This is the foundation of the famous indolic effect in perfumery-referring to ripe, slightly “overripe,” faintly corrupted yet highly erotic, animalic floral scents-making indole a core soul-material of white flowers such as jasmine, tuberose, orange blossom, lilac, and honeysuckle. Its chameleon-like role is also expressed through classic pairings: combined with benzyl acetate to reconstruct jasmine; paired with methyl anthranilate to evoke orange blossom and narcissus; or blended with alpha-terpineol to mimic lilac. Because it is extremely potent, highly diffusive, and offers moderate to fairly good persistence-and because its crystalline form is difficult to weigh accurately-perfumers often dilute indole into 1% or 10% solutions in ethanol, DPG, or benzyl benzoate to control dosage more precisely.
Applications
Indole is not only a crucial puzzle piece in floral and oriental perfumes, but also appears in trace amounts in food flavors to create cheese-like nuances or ripe-fruit effects. However, using indole in cosmetics requires careful consideration: it is more commonly found in cleansing products or dark-colored soaps rather than skin-whitening creams due to serious color-change issues. This is the most important caution, because indole is extremely sensitive to light and air, easily turning red or dark brown over time-especially when reacting with iron metals or certain aldehydes to form Schiff bases. Therefore, it should never be used in products that require a pure white appearance, and it must be stored in tightly sealed, dark-colored bottles.
From a safety standpoint, while indole is not banned by IFRA, its odor profile and stability naturally limit its practical use: perfumers typically keep it extremely low-from trace levels up to around 0.5% in a formula-to avoid an unpleasant fecal off-note. Indole’s signature is clearly present in celebrated masterpieces such as Joy by Jean Patou (rich natural jasmine), Carnal Flower by Frédéric Malle (emphasizing the fleshy side of tuberose), and Chanel No. 5 (used to balance a large aldehydic structure). In a classic reference work, Steffen Arctander described indole as powerfully suffocating and camphor-like, yet becoming pleasant, warm, and floral when diluted to extremely low levels below 0.1%; he also emphasized the recommendation to use it in 10% solution or lower to better manage its sensitivity to light and air.
4.97 / 5
(55) 55 total reviews
Share
Technical standards
Technical standards
| Physical appearance | Crystal | Conform |
| Color | White | Conform |
| Melting point | 50.0°C | Conform |
| Loss on drying | ≤ 0.5% | 0.34% |
| Purity | ≥ 99.0% | 99.17% |
Solubility @25˚C
Solubility @25˚C
| Solvent | Solubility (g/L) |
|---|---|
| ethanol | 491.84 |
| methanol | 742.52 |
| isopropanol | 276.23 |
| water | 3.45 |
| ethyl acetate | 113.83 |
| n-propanol | 375.24 |
| acetone | 201.69 |
| n-butanol | 324.73 |
| acetonitrile | 94.17 |
| DMF | 280.69 |
| toluene | 22.48 |
| isobutanol | 252.98 |
| 1,4-dioxane | 150.6 |
| methyl acetate | 84.08 |
| THF | 591.38 |
| 2-butanone | 156.95 |
| n-pentanol | 318.14 |
| sec-butanol | 269.04 |
| n-hexane | 37.64 |
| ethylene glycol | 97.61 |
| NMP | 129.02 |
| cyclohexane | 10.59 |
| DMSO | 250.83 |
| n-butyl acetate | 50.02 |
| n-octanol | 44.18 |
| chloroform | 132.6 |
| n-propyl acetate | 58.09 |
| acetic acid | 195.13 |
| dichloromethane | 124.52 |
| cyclohexanone | 106.64 |
| propylene glycol | 97.86 |
| isopropyl acetate | 51.62 |
| DMAc | 151.87 |
| 2-ethoxyethanol | 333.15 |
| isopentanol | 216.34 |
| n-heptane | 20.87 |
| ethyl formate | 80.6 |
| 1,2-dichloroethane | 84.61 |
| n-hexanol | 143.42 |
| 2-methoxyethanol | 431.59 |
| isobutyl acetate | 42.91 |
| tetrachloromethane | 25.53 |
| n-pentyl acetate | 48.68 |
| transcutol | 532.66 |
| n-heptanol | 58.29 |
| ethylbenzene | 15.32 |
| MIBK | 86.95 |
| 2-propoxyethanol | 213.34 |
| tert-butanol | 189.1 |
| MTBE | 140.21 |
| 2-butoxyethanol | 111.18 |
| propionic acid | 129.57 |
| o-xylene | 14.98 |
| formic acid | 146.54 |
| diethyl ether | 291.28 |
| m-xylene | 17.36 |
| p-xylene | 17.85 |
| chlorobenzene | 30.37 |
| dimethyl carbonate | 43.29 |
| n-octane | 7.86 |
| formamide | 241.33 |
| cyclopentanone | 195.51 |
| 2-pentanone | 161.38 |
| anisole | 56.91 |
| cyclopentyl methyl ether | 163.7 |
| gamma-butyrolactone | 159.49 |
| 1-methoxy-2-propanol | 264.57 |
| pyridine | 126.98 |
| 3-pentanone | 103.55 |
| furfural | 120.95 |
| n-dodecane | 4.83 |
| diethylene glycol | 157.78 |
| diisopropyl ether | 54.21 |
| tert-amyl alcohol | 175.95 |
| acetylacetone | 86.03 |
| n-hexadecane | 5.05 |
| acetophenone | 41.66 |
| methyl propionate | 103.5 |
| isopentyl acetate | 48.68 |
| trichloroethylene | 116.27 |
| n-nonanol | 43.12 |
| cyclohexanol | 97.11 |
| benzyl alcohol | 62.14 |
| 2-ethylhexanol | 62.31 |
| isooctanol | 48.9 |
| dipropyl ether | 115.28 |
| 1,2-dichlorobenzene | 25.93 |
| ethyl lactate | 35.57 |
| propylene carbonate | 61.99 |
| n-methylformamide | 227.55 |
| 2-pentanol | 206.89 |
| n-pentane | 36.33 |
| 1-propoxy-2-propanol | 120.42 |
| 1-methoxy-2-propyl acetate | 62.11 |
| 2-(2-methoxypropoxy) propanol | 94.29 |
| mesitylene | 11.62 |
| ε-caprolactone | 84.65 |
| p-cymene | 13.27 |
| epichlorohydrin | 212.34 |
| 1,1,1-trichloroethane | 46.32 |
| 2-aminoethanol | 224.26 |
| morpholine-4-carbaldehyde | 138.72 |
| sulfolane | 146.11 |
| 2,2,4-trimethylpentane | 12.2 |
| 2-methyltetrahydrofuran | 279.7 |
| n-hexyl acetate | 57.69 |
| isooctane | 9.87 |
| 2-(2-butoxyethoxy)ethanol | 151.76 |
| sec-butyl acetate | 41.7 |
| tert-butyl acetate | 56.89 |
| decalin | 7.62 |
| glycerin | 122.65 |
| diglyme | 220.98 |
| acrylic acid | 95.17 |
| isopropyl myristate | 31.45 |
| n-butyric acid | 181.3 |
| acetyl acetate | 38.43 |
| di(2-ethylhexyl) phthalate | 28.8 |
| ethyl propionate | 52.25 |
| nitromethane | 179.85 |
| 1,2-diethoxyethane | 175.43 |
| benzonitrile | 36.88 |
| trioctyl phosphate | 21.67 |
| 1-bromopropane | 88.93 |
| gamma-valerolactone | 167.62 |
| n-decanol | 33.45 |
| triethyl phosphate | 25.13 |
| 4-methyl-2-pentanol | 93.25 |
| propionitrile | 112.22 |
| vinylene carbonate | 56.67 |
| 1,1,2-trichlorotrifluoroethane | 99.39 |
| DMS | 40.77 |
| cumene | 15.21 |
| 2-octanol | 36.51 |
| 2-hexanone | 99.9 |
| octyl acetate | 36.12 |
| limonene | 22.54 |
| 1,2-dimethoxyethane | 256.79 |
| ethyl orthosilicate | 27.73 |
| tributyl phosphate | 27.27 |
| diacetone alcohol | 88.23 |
| N,N-dimethylaniline | 41.11 |
| acrylonitrile | 96.13 |
| aniline | 65.11 |
| 1,3-propanediol | 260.77 |
| bromobenzene | 22.08 |
| dibromomethane | 84.33 |
| 1,1,2,2-tetrachloroethane | 85.49 |
| 2-methyl-cyclohexyl acetate | 38.59 |
| tetrabutyl urea | 34.31 |
| diisobutyl methanol | 36.99 |
| 2-phenylethanol | 49.58 |
| styrene | 19.47 |
| dioctyl adipate | 39.12 |
| dimethyl sulfate | 49.35 |
| ethyl butyrate | 44.61 |
| methyl lactate | 57.61 |
| butyl lactate | 42.02 |
| diethyl carbonate | 28.77 |
| propanediol butyl ether | 75.77 |
| triethyl orthoformate | 36.59 |
| p-tert-butyltoluene | 13.38 |
| methyl 4-tert-butylbenzoate | 45.91 |
| morpholine | 254.1 |
| tert-butylamine | 127.93 |
| n-dodecanol | 27.07 |
| dimethoxymethane | 266.17 |
| ethylene carbonate | 48.28 |
| cyrene | 67.38 |
| 2-ethoxyethyl acetate | 77.39 |
| 2-ethylhexyl acetate | 36.8 |
| 1,2,4-trichlorobenzene | 29.93 |
| 4-methylpyridine | 79.05 |
| dibutyl ether | 70.68 |
| 2,6-dimethyl-4-heptanol | 36.99 |
| DEF | 141.36 |
| dimethyl isosorbide | 121.87 |
| tetrachloroethylene | 74.0 |
| eugenol | 58.49 |
| triacetin | 52.91 |
| span 80 | 68.34 |
| 1,4-butanediol | 136.82 |
| 1,1-dichloroethane | 70.18 |
| 2-methyl-1-pentanol | 208.92 |
| methyl formate | 102.72 |
| 2-methyl-1-butanol | 230.82 |
| n-decane | 7.96 |
| butyronitrile | 134.58 |
| 3,7-dimethyl-1-octanol | 49.46 |
| 1-chlorooctane | 23.8 |
| 1-chlorotetradecane | 11.23 |
| n-nonane | 7.8 |
| undecane | 5.87 |
| tert-butylcyclohexane | 10.16 |
| cyclooctane | 5.3 |
| cyclopentanol | 153.52 |
| tetrahydropyran | 205.54 |
| tert-amyl methyl ether | 110.19 |
| 2,5,8-trioxanonane | 161.41 |
| 1-hexene | 114.0 |
| 2-isopropoxyethanol | 191.49 |
| 2,2,2-trifluoroethanol | 49.7 |
| methyl butyrate | 65.54 |
Scent© AI
-
CAS NUMBER
120-72-9
-
FAMILIES
-
BRAND
Scent.vn
-
EVAPORATION RATE
Slow
-
Odor impact
High est.
-
FLASH POINT
387.7 ˚C est.
Olfactory Pyramid

Notes
| Floral |
| Animal |
| Pungent |
| Musty |
| Burnt |
| Maximum acceptable concentrations in the finished product (%) | |||
|---|---|---|---|
|
Category 1
Products applied to the lips
|
No restriction |
Category 7A
Rinse-off products applied to the hair with some hand contact
|
No restriction |
|
Category 2
Products applied to the axillae
|
No restriction |
Category 7B
Leave-on products applied to the hair with some hand contact
|
No restriction |
|
Category 3
Products applied to the face/body using fingertips
|
No restriction |
Category 8
Products with significant anogenital exposure
|
No restriction |
|
Category 4
Products related to fine fragrance
|
No restriction |
Category 9
Products with body and hand exposure, primarily rinse off
|
No restriction |
|
Category 5A
Body lotion products applied to the body using the hands (palms), primarily leave on
|
No restriction |
Category 10A
Household care products with mostly hand contact
|
No restriction |
|
Category 5B
Face moisturizer products applied to the face using the hands (palms), primarily leave on
|
No restriction |
Category 10B
Household care products with mostly hand contact, including aerosol/spray products (with potential leave-on skin contact)
|
No restriction |
|
Category 5C
Hand cream products applied to the hands using the hands (palms), primarily leave on
|
No restriction |
Category 11A
Products with intended skin contact but minimal transfer of fragrance to skin from inert substrate without UV exposure
|
No restriction |
|
Category 5D
Baby Creams, baby Oils and baby talc
|
No restriction |
Category 11B
Products with intended skin contact but minimal transfer of fragrance to skin from inert substrate with potential UV exposure
|
No restriction |
|
Category 6
Products with oral and lip exposure
|
No restriction |
Category 12
Products not intended for direct skin contact, minimal or insignificant transfer to skin
|
No restriction |
Shipping & Returns
-
 All orders will be processed within 1-2 business days from the time the order is confirmed.
All orders will be processed within 1-2 business days from the time the order is confirmed. -
 Free shipping is available for orders valued at $200 or more.
Free shipping is available for orders valued at $200 or more. -
 Delivery time is 1-3 business days for local areas, 3-7 days for suburban and nationwide deliveries, and 1-4 weeks for international orders.
Delivery time is 1-3 business days for local areas, 3-7 days for suburban and nationwide deliveries, and 1-4 weeks for international orders. -
 You have 30 days from the date of receipt to initiate the return process.
You have 30 days from the date of receipt to initiate the return process.
Certificates of Quality
-
Certificate of Analysis (COA)
Provides information on the physical and chemical properties of the product.Download -
IFRA Certificate of Conformity
Sets safety standards and guidelines for the product in manufacturing.Download -
Safety Data Sheet (SDS)
Provides important safety guidelines for transporting, storing, and using the product.Download








