Essential oils are called “essential” because they come from the essence of a plant. They are distillations of molecules called terpenes—also known as hydrocarbons—which provide a plant with its scent. Many essential oils also contain terpenoids, which are chemicals that also influence the scent, color, and flavor of the plant, and phenylpropanoids, which protect the plant from ultraviolet light and creatures that eat plants, and aid in the attraction of pollinators (bees and butterflies) by contributing to scent and pigment. The way a plant smells can help protect it from pests while attracting pollinators by releasing its scent into the air at the warmest time of day during the growing season. This is one of the reasons that flowers smell so good in spring and early summer. Some plants release their scent at night to attract moths and bats that also serve as pollinators.
Plants store their essential oil in structures throughout the plant, including glands, conduits, and cavities from which they can secrete the liquid in microscopic drops during pollination season. While we tend to think of plants producing scent within their flowers, the oil producing the aroma may actually be found in the stem, leaves, fruit, bark, and even the roots of the plant, creating quite a powerful system with which to attract bees, moths, hummingbirds, and other active pollinators.
These oils are gathered from a wide range of plants. More than 17,500 plant species produce an essential oil, though hardly more than 1 percent of these are harvested for their oil. The essential oils we can buy today come from plants that can be grown on farms—in other words, they thrive well in agricultural conditions where the potential for drought, insect infestations, and disease can be controlled. These plants also produce their oil fairly easily and dependably using one or more of the methods described later in this book (see How are essential oils obtained from plants?). Some plants do not surrender their oil using one of these methods, while others are too delicate to withstand the extraction process. Not all plants have a pleasant scent or taste, so the effort of cultivating them and extracting their oil may not be worthwhile.
Many purveyors of essential oils tout their medicinal properties beyond their scents. With more than 350,000 plant species identified around the world, however, only a tiny fraction has been assessed for their potential in healing or combating disease. Science has gravitated to the essential oils already on the market, as the claims of their usefulness as medicine have been passed down through more than a century of common usage. These familiar oils also have the advantage of being readily available for testing.
Today more than 100 essential oils are available commercially, obtained from showy flowering plants like rose, geranium, jasmine, and bergamot; from spice plants like cinnamon, ginger, and clove; from herbs like basil, oregano, and thyme; and from citrus fruits including orange, lemon, and tangerine.
Calling these substances “oils” is a little misleading, as they are not oils in the same way as canola, olive, or vegetable oil are. Oils used in cooking are known as fixed oils, because they do not change their state when heated. Canola oil, for example, continues to be a liquid even at high heat, because it contains fatty acids that prevent it from vaporizing.
Essential oils, however, are volatile oils, because they turn from liquid to gas as they get warm. This makes them especially appropriate for use in aromatherapy, as they can be warmed, vaporized, and inhaled easily.
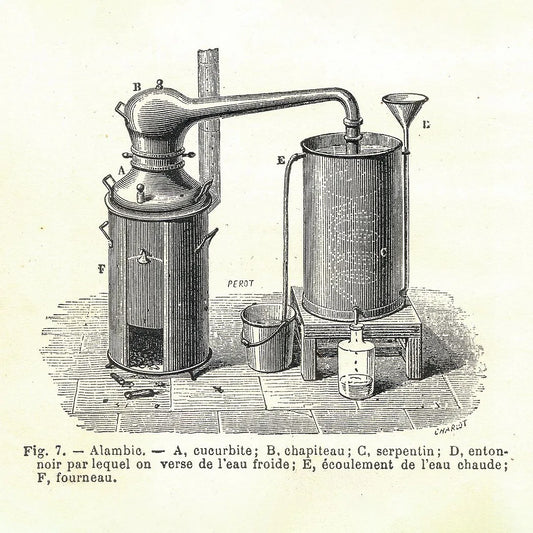
Essential oils are obtained from plants using one of three methods: distillation, expression, or extraction, either using a solvent or a process called hypercritical CO2 extraction. (See How are essential oils obtained from plants?)