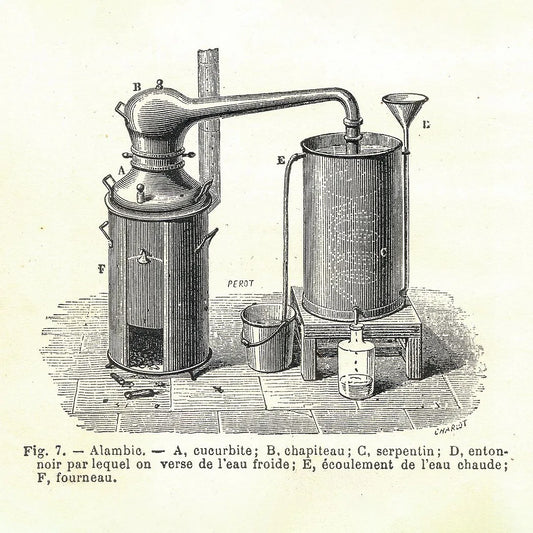
Three basic methods are used to extract essential oils from plants: distillation, expression, and either solvent extraction or hypercritical CO2 extraction. The most widely used method, involving both water and steam, dates back to 1928 when Joseph Franklin Clevenger published instructions for constructing an apparatus “for the determination of volatile oil,” involving a flask to hold the plant material and some water, a separator to isolate the oil from the water in a graduated tube, and a condenser to gather the product of this process. Clevenger’s steam distillation process replaced the previous, more cumbersome method of boiling the plant material in water and waiting until the plant’s oil surfaced and formed its own layer on the water—a time-consuming endeavor at best. The Clevenger apparatus proved fragile, and correct placement of the separation valve became tricky, so in 1951, Jakub Deryng made modifications to the design and published his method. The Deryng design, with some modifications, remained in use into the twenty-first century, though much more modern techniques now include the use of microwaves and supercritical fluids—fluids like carbon dioxide and water, which have properties of both a liquid and a gas.
Distillation uses steam to deconstruct the plant and capture the vapor that rises from it. The vapor contains the terpenes than give the plant its scent. The plants stem, leaves, flowers, roots, or bark may be used in the process. Several different kinds of distillation are used for different plants, but they all involve placing the plant matter on a grid or screen in a sealed container so moisture can surround the material.
Water distillation places the plant directly in water inside the container and boils the water until it produces steam. The steam permeates the plant, causing it to fall apart and release the terpenes. This method is especially effective with flower blossoms, which are delicate and break down easily when steamed.
Water-and-steam distillation is less direct, placing the plant on the screen but keeping the water below it. The steam rises and penetrates the plant, capturing the terpenes as it elevates within the container.
Steam distillation eliminates the water inside the container; instead, the steam is injected into the container at high pressure from the bottom. As the steam rises, the plant comes apart, just as it does in the more direct versions of this process.
Hydrodiffusion distillation injects the steam from the top of the container instead of the bottom. This is particularly good for extracting the essence from tough parts of the plant like bark or a woody trunk.
In all these methods, the vapor that rises from the plant gets channeled into a condenser, where the steam cools. The resulting liquid contains water from the distillation process and the essential oil, which is lighter than water and floats on top of it. This makes it easy to capture by siphoning off the oil.
Oils collected from fruit rind or pulp are gathered using expression, sometimes described as cold-pressing. This mechanical process does not use heat but involves turning and puncturing the fruit in a machine and collecting the juice and essential oil beneath it. This allows the essential oil to rise to the top of the liquid, leaving the juice behind. Lemon, orange, lime, tangerine, and other oils pressed from fruits are obtained in this manner.
The third method, solvent extraction, is used when the plants themselves are too fragile to survive the steam pressure involved in distillation or the mangling that happens during expression. Fragile flowers like jasmine and gardenia would not yield their oils using these methods, so bottlers use solvents to extract the terpenes and chlorophyll from these plants, as well as some solid plant matter and wax that reside within the plant. This results in a compound called a concrete—having nothing to do with cement—which is then combined with alcohol to create an absolute, a scented material for specific uses like perfumes and cosmetics. Solvents involved in this method may include ethanol, methanol, hexane, or petroleum ether. These are removed at the end of the process, however, leaving just 5 to 10 parts per million (ppm) of residue from the solvent. Essential oils extracted in this manner are not appropriate for topical use or ingestion; they usually are used only for aromatherapy or as ingredients in nonedible, scented products.
Hypercritical CO2 extraction uses carbon dioxide at very high pressure, transforming liquid into gas until there is no distinction between the two. The substance that results from this can pass through the plant matter and carry out the essential oil with it. This fairly new method has been in use in other industries, especially in removing caffeine from coffee beans.